Point of Product
Features and Benefits
| Features: | Benefits: |
| Allows for the sterile connection of two fluid paths in a uncontrolled environment | Elimination of the need for laminar air flow hoods and glove boxes to carry out sterile connections |
| Genderless design | Reduction of part numbers and simplification of single-use systems |
| Operating temperature range 2°C to 60°CStorage to -80°CPressure rating from up to 3 barg for 90 days4 barg for shorter periods | Can be used in a wide variety of applications - including tangential flow filtration (TFF) with pulsated 4 barg range |
| Operating pH range from 2-12Compatible with Dimethylacetamide (DMA) & Dimethyl Sulfoxide (DMSO) | Can be used in a wide range of processes, including antibody drug conjugates |
| 6.35 mm (¼ in.), 9.53 mm (⅜ in.), 12.7 mm (½ in.), 15.8 mm (⅜ in.), 19 mm (¾ in.). in hose barb and 12.7 mm (½ in.). Sanitary connections | Wide range of connection sizes allows handling of different volumes - universal face allows for step up and step down connections |
| Tamper resistant caps on devices | Enchance end user confidence between point of manufacturer and point of use |
| Intuitive operation | Clear visual indication for end user makes the sterilie connection the correct way 100% of the time |
| Each size has a different colored cap | Clear visual indication for end user on sizes being used |
| BPA-free PES used as material of construction | Eliminates any BPA toxicological concerns |
| PES material compliant to USP 87, 88, 661, 85, 788 | In line with regulatory expectations |
| Each device individually marked with batch number and serial number | Device traceability that is linked to the 100% on-line inspection at point of manufacture |
| Vision systems on 100% of devices during manufacturing | Vision system that is capable of detecting membrane and weld defects to ensure absence of critical defects |
Quality Standards
| Manufactured under a quality management system certified to ISO9001 |
| Manufactured in a clean room Class 7 in operation |
| Supplied with a certificate of test confirming the quality standards and quality control tests performed by Pall |
| Each connector is individually marked with batch number and serial number |
|
Batch release criteria:
|
|
Device release criteria:
|
|
The fluid path materials of construction have been tested and meet the regulatory requirements of:
|
| The fluid path materials of construction do not contain substances derived from animal products (i.e. BSE/TSE risk free) |
Specification
Materials of Construction
| Product Contract Parts | Material of Construction |
| Connector body | Polyesthersulfone (PES) |
| O-ring | Platinum-cured silicone |
| Protective cap | Thermoplastic elastomer |
| Cover and tab | Polybutylene terephtalate (PBT) |
Sterilization Specifications
| Sterilization Method | Guidance |
| Gamma irradiation | Maximum 50 kGy |
| Autoactive | One 75 minute cycle at 130°C |
Operating Specifications
| Parameter | Value |
| Up to 3 barg at 2-60°C | Up to 50 kGy |
| Up to 4 barg at 2-60°C | Up to 2 days (48 hours) |
Nominal Dimensions
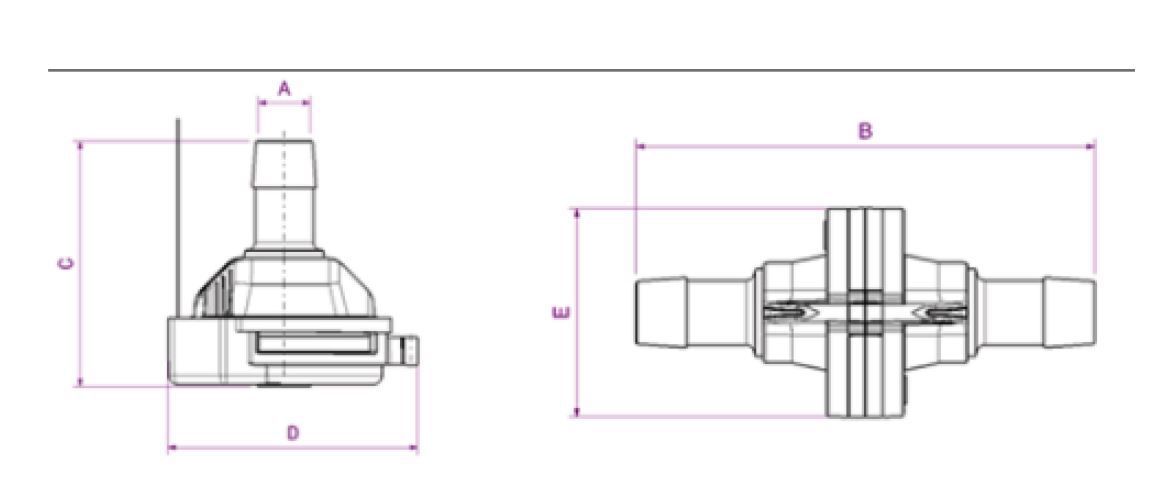
| Part Number | PSC1G07 | PSC1G10 | PSC1G06 | PSC1G11 | PSC1G08 | PSC1G05 |
| Size |
6.35 mm
(¼ in.)
Hose barb
|
9.53 mm
(⅜ in.)
Hose barb
|
12.7 mm
(½ in.)
Hose barb
|
15.88 mm
(⅜ in.)
Hose barb
|
12. 7 mm
(½ in.)
Sanitary
|
19 mm
(¾ in.)
Hose barb
|
| Internal diameter (A) | 6 mm (0.2 in.) |
8 mm
(0.3 in.)
|
14 mm
(0.6 in.)
|
14 mm
(0.6 in.)
|
13 mm
(0.5 in.)
|
14 mm
(0.6 in.)
|
| Length when actuated (B) |
97 mm
(3.8 in.)
|
97 mm
(3.8 in.)
|
108 mm
(4.3 in.)
|
120 mm
(4.7 in.)
|
99 mm
(3.9 in.)
|
140 mm
(6 in.)
|
| Connector length with cap (C) |
58 mm
(2.3 in.)
|
58 mm
(2.3 in.)
|
64 mm
(2.5 in.)
|
70 mm
(2.8 in.)
|
59 mm
(2.3 in.)
|
79 mm
(3 in.)
|
| Maximum diameter of connector with cap (D) |
70 mm
(2.8 in.)
|
70 mm
(2.8 in.)
|
70 mm
(2.8 in.)
|
70 mm
(2.8 in.)
|
70 mm
(2.8 in.)
|
70 mm
(2.8 in.)
|
| Maximum diameter or connected device (E) |
49 mm
(1.9 in.)
|
49 mm
(1.9 in.)
|
49 mm
(1.9 in.)
|
49 mm
(1.9 in.)
|
49 mm
(1.9 in.)
|
49 mm
(1.9 in.)
|
| Weight | 33 g | 33 g | 34 g | 36 g | 36 g | 41 g |
Applications
| Media Preparation and transfer |
| Buffer preparation and transfer |
| Transfer of inoculum to bioreactor |
| Sampling during fermentation / cell culture |
| Bioreactor harvest |
| Sterile fluid transfer between unit operations |
| Bulk handling of sterile material in non-classified environments |
| Probe insertion into bioreactors, mixers and 3D biocontainers |
| Sterile filtration manifolds |
| Hybrid stainless steel and single-use system connection |
| Connection of bulk sterile material to filling machine |
| Sterile waste removal from process streams |

